

Metal on Metal Hip Replacement Lawsuits & Settlements
Metal on Metal Hip Replacement Lawsuits
If you got a metal-on-metal hip replacement, you may be at risk for serious health complications caused by toxic metal debris. Thousands of patients have already taken legal action, filing metal hip replacement lawsuits against manufacturers for failing to warn about these dangers.
How Metal on Metal Hip Replacements Can Cause Harm
- Toxic Metal Exposure: As the metal components rub together, tiny metal particles shed into the bloodstream, leading to metallosis, severe inflammation, and tissue destruction.
- High Failure Rates & Revision Surgeries: Many people need early replacement surgery due to pain, swelling, or implant failure, putting them through another round of major surgery.
Which Metal Hip Replacements Are Involved in Lawsuits?
Several major hip replacement manufacturers have been sued for making and selling defective metal-on-metal hip implants, including:
- Johnson & Johnson DePuy Pinnacle Hip – Lawsuits claim the company knew the risks but continued selling the device.
- Biomet M2a Magnum Hip – Patients have reported severe tissue and bone damage, requiring additional surgery.
- DePuy ASR Hip – Recalled after thousands of reports of device failure and serious complications.
Why Are People Filing Metal Hip Replacement Lawsuits?
Patients who received metal-on-metal hip implants are taking legal action to hold manufacturers accountable for:
- Selling defective medical devices without proper safety testing.
- Failing to warn patients about the risks of metallosis, metal toxicity, and early implant failure.
- Negligence in designing and marketing unsafe implants.
Many metal on metal hip replacement lawsuits have already resulted in multi-million dollar verdicts and settlements, and litigation is still ongoing.
Why Choose mctlaw for Your Metal Hip Replacement Lawsuit?
- We filed the first Metal Hip Replacement Lawsuit in the U.S., setting the stage for legal action against implant manufacturers.
- Decades of experience holding medical device companies accountable for unsafe products. Our lawyers have a deep understanding of orthopedic implants, medical complications, and legal strategies.
- No upfront legal fees: we only get paid if you win your case.
Do You Qualify for a Metal Hip Replacement Lawsuit?
We would like to review your case at no cost if you match these criteria:
- You have a Biomet, DePuy Pinnacle, or DePuy ASR metal on metal hip implant.
- You had a revision surgery to remove and replace the metal on metal hip implant, or
- You are scheduled for an upcoming revision surgery, or
- Your surgeon advised that revision surgery is too risky due to your current health.
How to Identify Your Metal-on-Metal Hip Implant
If you don’t know the specific type of metal-on-metal (MoM) hip implant you have, here’s how you can find out:
Contact Your Surgeon or Hospital
Ask for the Implant Record: Also known as the “device sticker page,” this record contains the manufacturer’s label with essential information about your implant.
Request Your Operative Report: This document details the surgery performed and often specifies the implant used.
For a detailed walkthrough of this process, download our comprehensive guide: “How to find out what kind of hip replacement you have”
Identifying your specific hip implant is essential to know what legal options available to you.

Click Here To See a List of Revision Surgeons Listed by State
What’s Wrong with Metal-on-Metal Hips?
Metallosis
Metal-on-metal hip implants can release toxic metal particles into your body every time you move, leading to metallosis, a serious form of heavy metal poisoning. This can cause bone loss, nerve damage, and muscle destruction around the hip joint.
No Symptoms
Many metal-on-metal hip patients report feeling fine and having no symptoms, but blood tests reveal dangerous metal poisoning. Find out why early detection is critical and how often experts say you should get blood testing.
Revision Surgery
Metal-on-metal hip implants usual require revision hip surgery to remove and replace the defective parts. Not all surgeons perform this complex procedure. Learn more about finding the right revision surgeon and check out our list of hip revision surgeons across the country.
Questions About Filing a Lawsuit:
How do I know if I have a case?
Our Firm will review your information to determine if we can represent you. We might even be able to review some of your medical information over the phone. Often, we need the following documentation:
- Orthopedic surgeon’s office chart
- Operative reports for the original joint replacement surgery and the revision (if performed)
- Device record for the original surgery from the hospital chart (this shows the product and lot numbers of the components of the device)
We can help you track down many of these records. Once our attorneys complete your review, they’ll tell you if we can represent you in your case. However, due to the unique complexity of each case, there’s no one formula to determine if you do or don’t have a viable case.
Why mctlaw does NOT sue orthopedic surgeons:
We do not represent patients in lawsuits against orthopedic surgeons because, in defective hip replacement cases, the problem is with the device, not the doctor. Often your orthopedic surgeon can actually HELP your case.
We believe the companies that made the hip replacements misled surgeons about the safety of their products. That’s why we limit our representation strictly to prosecuting manufacturers, sellers, and distributors of defective joint replacements.
Can you represent me even if I don’t live near your offices?
Yes! Our lawyers represent people across the entire United States. We are a nationwide litigation firm with regional offices in Washington DC, Seattle, WA, and Sarasota, FL.
When it comes to hiring an attorney, make your choice based on experience in this specific field. These are high-profile product liability cases. Overall, the law firm you hire should have the resources to manage your case against huge multinational corporations. This is not a typical personal injury case and a local attorney may not be equipped to fight your case.
Is there a deadline to file a defective hip replacement lawsuit?
Yes, definitely. There is a limited period of time to file a lawsuit for injuries suffered as the result of a defective product. Therefore, do not delay in getting more information because it could hurt your case.
Do you collect and refer cases? Because I don’t want that.
No. Absolutely not. mctlaw is not a referral mill or case collection firm. If we take your case, we represent you. Our attorneys represent injured clients across the country in defective product liability cases. Our attorneys and staff are your direct contact in all aspects of your case.
How much does it cost to hire mctlaw for a metal on metal hip replacement case?
There is no cost unless we recover money from a verdict or a settlement. Our Firm charges a percentage of any settlement or judgment. In other words, if you do not get any compensation at the end of the lawsuit, we get no fee and you owe us nothing.
Why hire the attorneys at mctlaw?
Mctlaw has focused its practice on defective hip replacement cases for over a decade. Our Firm filed the first lawsuit involving a modern generation model metal on metal hip replacement in the United States in 2008. Find out what kind of experience an attorney has in litigating orthopedic joint replacement cases.
Complications From Metal on Metal Hip Replacements
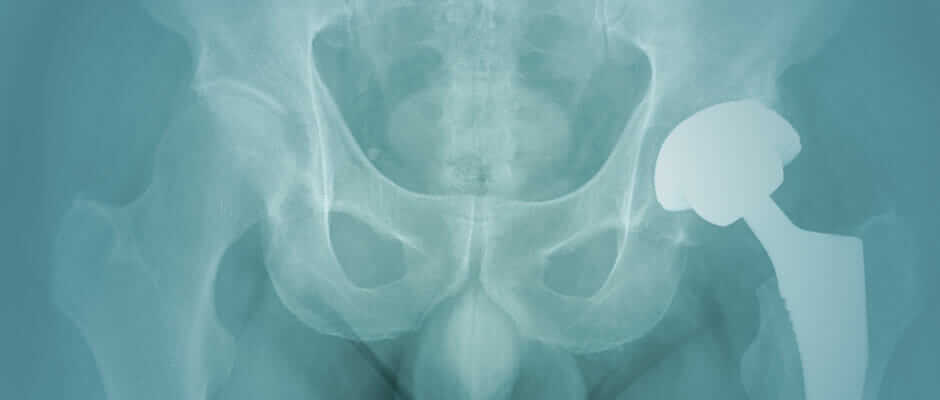
pseudotumors
A pseudotumor is a large, solid, or semi-liquid mass of soft tissue growth inside the body, usually around the hip joint. They are associated with metal on metal hip replacements, and typically form as a negative reaction to the metal debris.

cobalt chromium toxicity
Cobalt and chromium metal debris from metal on metal hip replacements can end up in a patient’s bloodstream. This may trigger a hypersensitive immune response in their body.

metallosis
A type of metal poisoning that involves a build-up of metal debris in the body’s soft tissue.

revision surgery
Metal-on-Metal hip implants are known to have some negative side effects. One of the ways to reverse the side effects is to remove and replace the bad hip implant. This is called a revision surgery.
Click on Each State To View the Number of Cases
The map below shows the number of people across the country with hip replacement cases that we currently represent or have represented in the past. This is not a comprehensive list as we’re reviewing patients’ medical records and accepting metal-on-metal hip cases every day from all 50 states.
Frequently Asked Questions:
What Types of Hip Replacements are Recalled?
The DePuy ASR and Exactech hip implants have been recalled. In addition, many companies simply stopped making and selling metal on metal hip replacements instead of recalling them. The Zimmer Biomet Magnum M2a and the Johnson & Johnson DePuy Pinnacle metal on metal hips were pulled from the market but never recalled.
Mctlaw currently litigates the following recalled hips:
How Do I Find Out What Kind of Hip Replacement I Have?
To find out what kind of hip replacement you have, start by calling your doctor or hospital and ask for a copy of the operative report and the implant record. Sometimes you may have to fill out a medical records request form or send a letter asking for your records. We’ve created a simple guide to finding out what type of hip you have to help make the process easier for you.
How Do I Know if I Have a Hip Replacement Case?
It depends on many circumstances, including type and brand of hip implant, date of implant, if you’ve had revision surgery or your doctor recommends revision surgery. There are many factors that go into a case review. Our legal team needs to review some of your medical information to determine if we can represent you. We will ask for the following documents:
- Orthopedic surgeon’s office chart
- Operative reports for the original joint replacement surgery and the revision (if performed)
- Device record for the original surgery from the hospital chart (this shows the product and lot numbers of the components of the device).
We can help you track down many of these records. Once our legal team completes your review, they’ll tell you if we can represent you in your case. However, due to the unique complexity of each case, there’s no one formula to determine if you do or don’t have a viable case.
Will Lawyers Sue my Surgeon for a Bad Hip Replacement?
We do not represent patients in lawsuits against orthopedic surgeons because, in defective hip replacement cases, the problem is with the device, not the doctor. Often your orthopedic surgeon can actually HELP your case. We believe the companies that made the hip replacements misled surgeons about the safety of their products. That’s why we limit our representation strictly to prosecuting manufacturers, sellers, and distributors of defective joint replacements.
Can You Represent Me in a Hip Replacement Lawsuit if I Don’t Live Near One of Your Offices?
Yes! We can represent you in a defective hip replacement case anywhere in the country. We are a national law firm and our attorneys represent clients across the country. Our website includes a map of our hip replacement cases broken down by state.
Are you Going to Refer my Hip Replacement Case to Another Firm?
No. Mctlaw is not a referral mill or case collection firm. If we take your case, we represent you. We may refer you to other trusted attorneys, with your permission, if your hip is a brand that we are not actively litigating. But mctlaw is not a referral service. We are on leadership positions in various hip litigations and represent clients across the country in defective hip replacement lawsuits.
What is the Cost of a Hip Replacement Lawsuit?
There is no cost unless we recover money from a verdict or a settlement. Our Firm charges a percentage of any settlement or judgment. In other words, if you do not get any compensation at the end of the lawsuit, we get no fee and you owe us nothing.
Is there a Deadline to File a Hip Replacement Claim?
Yes, there is a deadline to file a hip replacement claim, but it depends on many factors, including your state or where you had the surgery. There is no one answer to this question. That’s why we offer a free case evaluation so we can review your basic medical records and determine if we think you have a viable claim. Do not delay getting more information about filing a lawsuit because you could run out of time.
Why Hire Our Attorneys to Represent You in a Hip Replacement Case?
Mctlaw has focused its legal practice on defective hip replacement cases for more than 15 years. Many law firms refer metal on metal hip cases to the attorneys at mctlaw rather than litigating these cases themselves. In 2008, our attorneys filed the first lawsuit in the United States involving a modern generation model metal on metal hip replacement. Attorneys at mctlaw hold leadership positions in ongoing hip implant litigation. Patients in jury trials across the country have won multimillion-dollar judgments for injuries caused by defective metal on metal hip replacements. Mctlaw is one of the few law firms leading these litigations nationally.
What are the Most Common Complications from Metal on Metal Hip Replacements?
Metallosis, pseudotumors, high cobalt chromium levels, revision surgery to remove and replace a bad hip implant. Metallosis is when small particles of metal rub off from the hip replacement and remain in the bloodstream. The metal particles can rot the healthy hip tissue around the implant. Pseudotumors are another reaction to metal particles. They are large, solid, or semi-liquid masses of soft tissue growth inside the body, usually around the hip joint. Cobalt-Chromium poisoning is also a complication of metal on metal hip replacements, and blood tests must be performed to assess this.
What are the Symptoms of a Failed Hip Replacement?
Many patients have no physical symptoms at all but have dangerously high levels of metal in their blood. Symptoms that you could experience include pain, squeaking in the joint, dislocation, and more. It’s important to know that you may not have any symptoms at all as the hip replacement fails. You should consult your orthopedic surgeon if you have any concerns about your hip implant.
Connect with us:
Content Reviewed by Michael Cowgill – Product Liability Lawyer

Michael Cowgill is an experienced attorney in the product liability division at mctlaw. Michael focuses his practice on defective medical devices such as recalled metal-on-metal hips and wrongful death lawsuits involving Kratom. Mr. Cowgill graduated Magna Cum Laude from Lewis & Clark Law School in Portland, OR. He volunteers as a high school mentor with a program for underprivileged youths interested in pursuing a future legal career.
This page was last updated:


Find out right now if you have a claim
Your case review is free. Don’t wait to get help because it could hurt your case.

As an experienced leader in these types of lawsuits, we were confident the firm would have the expertise. However, what surprised us most was the high level of excellent customer service from the firm’s staff!
Pat R.
I can’t recommend this firm enough. They have an outstanding team that truly care for their clients…I have been awarded a fair six figure settlement.
Nate M.
When I say “they went to bat” for me…this Law Firm literally did just that. They persevered to bring the hard-nosed Manufacturer to settle and provide me some recompense for everything I had to endure which led to this suit.
Me’Chelle


