Inspire Sleep Apnea Device Recall
Inspire Medical Recalls Sleep Apnea Implants Because of Dangerous Electrical Malfunctions
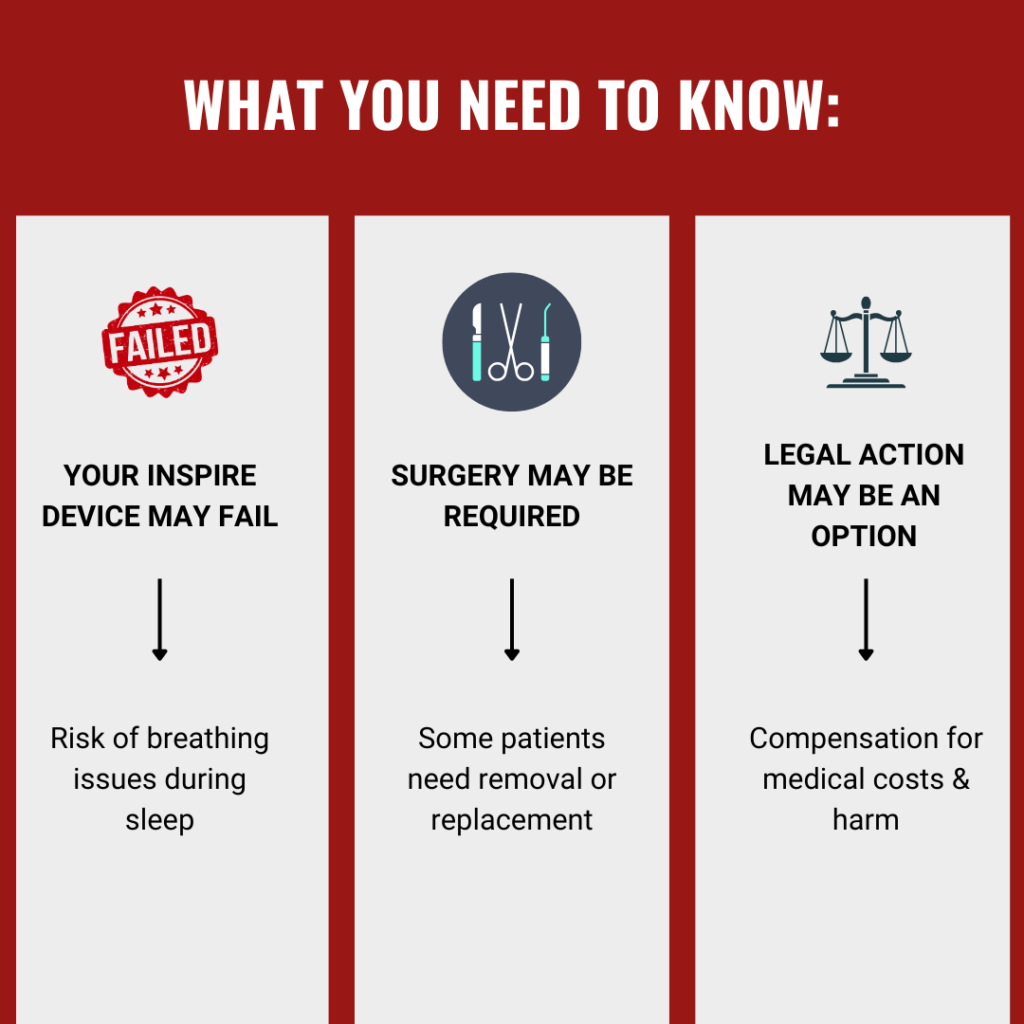
Inspire Medical has recalled its nerve-stimulating sleep apnea implant because of a serious electrical issue. Patients have experienced device failures, forcing them to undergo a revision surgery to remove or replace the implant. If you needed surgery because your Inspire implant failed, you may qualify for compensation.
Why Did Inspire Recall Its Sleep Apnea Implants?
Inspire recalled these devices because of an electrical leakage that can cause:
- The batteries to drain too quickly
- The device to stop working
- Electrical shocks to the user
The FDA issued a Class I recall, the most serious type, because the malfunction could cause severe health complications or death.
What Should You Do If Your Inspire Sleep Apnea Implant Fails?
When medical devices fail, the companies that make them can and should be held accountable. Mctlaw has decades of experience fighting for patients in medical product liability cases. If your Inspire sleep apnea implant failed and you needed revision surgery or suffered another injury, contact us now for a free case review. Our attorneys are actively reviewing cases.
If your Inspire sleep apnea implant failed, contact mctlaw today. We will review your case for free–there’s no cost and no pressure.
A faulty sleep apnea implant can be life-threatening. People with obstructive sleep apnea (OSA) rely on these devices to keep breathing while they sleep. Without treatment, OSA increases the risk of:
- Cardiac arrest
- High blood pressure
- Diabetes
- Pulmonary hypertension
- Stroke
- Dangerous heart rhythms
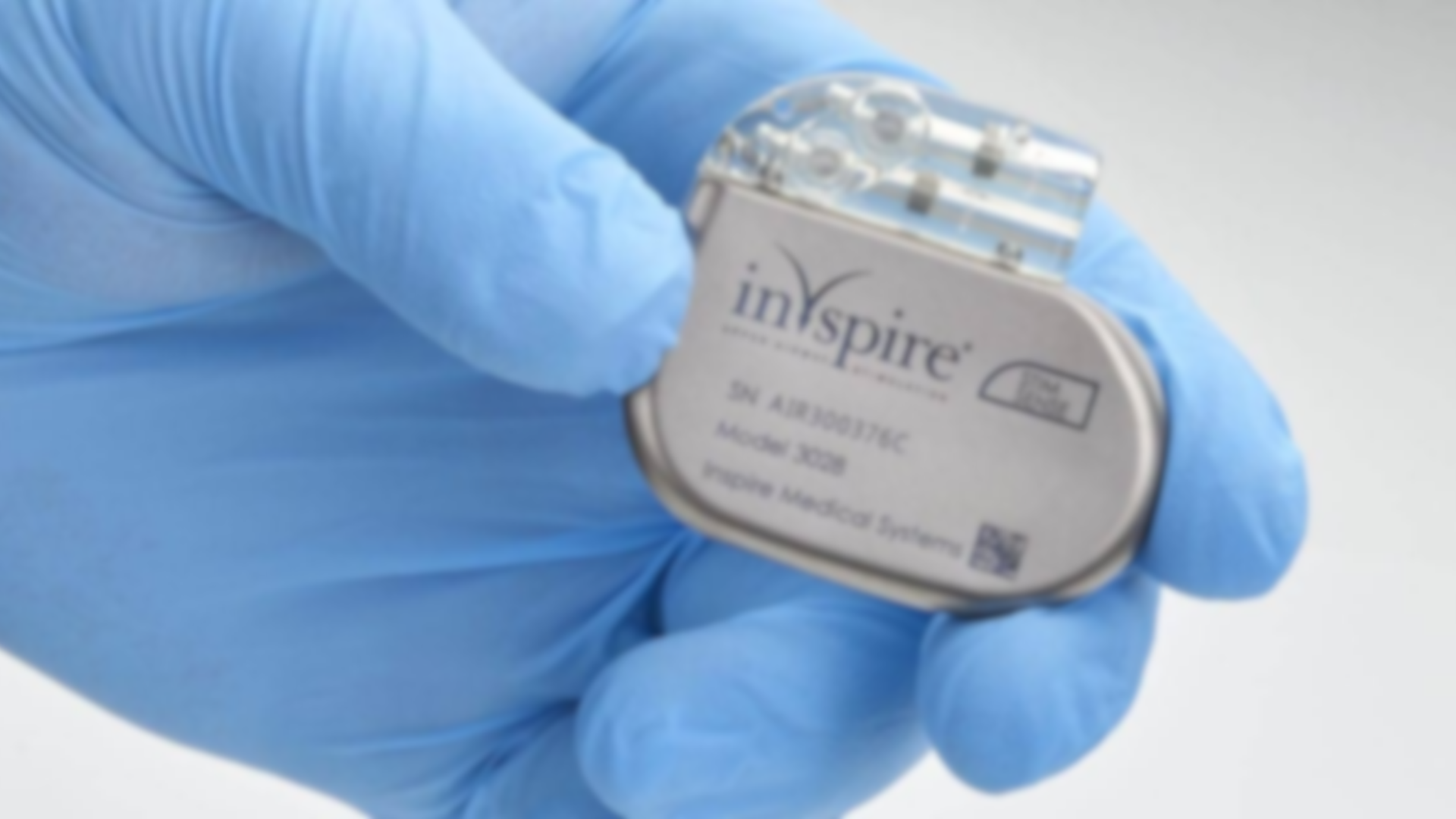
What Is the Inspire Sleep Apnea Implant?
Doctors surgically implant this device to treat obstructive sleep apnea (OSA), a condition that causes repeated breathing interruptions during sleep. OSA happens when soft tissue in the throat collapses, partially blocking the airway with the tongue.
The Inspire implant sends electrical pulses to airway muscles, moving the tongue out of the way so users can breathe. Doctors place the device near the collarbone during surgery. This treatment is meant for patients who haven’t had success with CPAP machines.

Content Reviewed by Altom Maglio – Attorney

Altom M. Maglio represents individuals across the United States in complex civil litigation, including COVID eviction moratorium takings claims, rails to trails conversions, listeria outbreak lawsuits, defective orthopedic devices and product liability lawsuits. Mr. Maglio founded mctlaw in 1999 to represent people in their fight against huge corporations and large government programs. He is also an active leader in the U.S. Court of Federal Claims Bar Association and the American Association for Justice Leadership Forum.
This page was last updated on:


Find out right now if you have a vaccine injury claim
Your Case Review is Free. Don’t Wait to Get Help Because There is a Deadline.
The COVID-19 Vaccine is NOT Eligible

As an experienced leader in these types of lawsuits, we were confident the firm would have the expertise. However, what surprised us most was the high level of excellent customer service from the firm’s staff!
Pat R.
I can’t recommend this firm enough. They have an outstanding team that truly care for their clients…I have been awarded a fair six figure settlement.
Nate M.
When I say “they went to bat” for me…this Law Firm literally did just that. They persevered to bring the hard-nosed Manufacturer to settle and provide me some recompense for everything I had to endure which led to this suit.
Me’Chelle